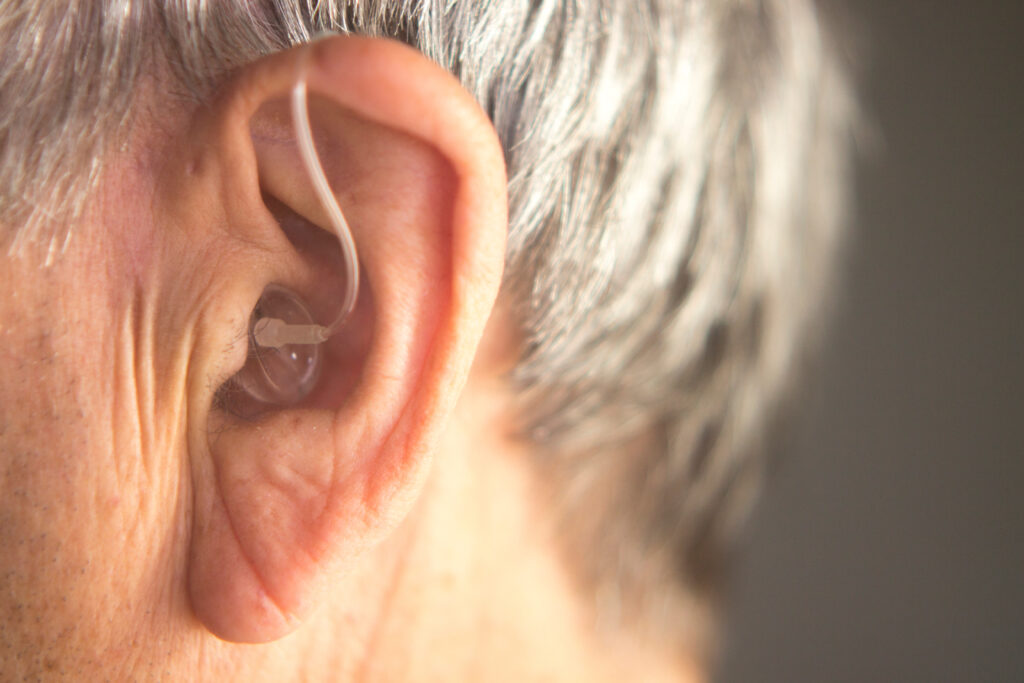
Some people avoid purchasing hearing aids because of their hefty price tags. The cost for a single hearing aid ranges from hundreds of dollars to more than $4,000. Moreover, Medicare and most private insurers don’t usually cover the expense. Thus, affordability is a “significant barrier” to purchasing hearing aids, according to a paper in the Hearing Journal, a hearing health care publication.
However, an FDA rule is slated to take effect in mid-October, at which point hearing aid manufacturers will have 240 days to amend relevant product labels and marketing to comply with the new OTC requirements. OTC hearing aids will likely be more affordable and accessible to consumers than most other FDA-approved hearing aids on the market right now.
Forbes’ recent article entitled “FDA Rule Allows Over-The-Counter Hearing Aids To Hit Shelves As Soon As October, Improving Access Nationwide” reports that according to the FDA’s new rule, over-the-counter (OTC) hearing aids are hearing aids intended for people at least 18 years old with perceived mild to moderate hearing loss.
Hearing aids will be available at stores and online retailers (who aren’t required to be licensed sellers) without the need for a medical exam, prescription or fitting adjustment by an audiologist or hearing health professional. The OTC hearing aids must be controllable by the user and customizable to the user’s hearing needs, allowing them to make volume and frequency-dependent changes based on their preferences without the assistance of a professional.
Note that OTC hearing aids are different from personal sound amplification products (PSAPs), which are used to amplify sounds in certain environments and aren’t subject to FDA regulation.
While specific cost information hasn’t been announced by the FDA, OTC hearing aids are expected to be more affordable than prescription hearing aids. Those are frequently sold bundled with audiology services. Affordable OTC hearing aids have the potential to make hearing aids more easily available to people with some degree of hearing loss who may not otherwise be able to afford them. Users also won’t be required to present a prescription from an audiologist or other hearing health professional to get them.
However, members of some hearing health industry associations are concerned about consumers purchasing and using OTC hearing aids without first completing a hearing evaluation conducted by a hearing health professional.
They worry people might damage their ears from overamplification or simply not get a positive result with the products and give up on hearing aids altogether. That has many social and health implications.
However, the Hearing Loss Association of America (HLAA) openly supports a regulated market for OTC hearing aids.
Reference: Forbes (Aug. 16, 2022) “FDA Rule Allows Over-The-Counter Hearing Aids To Hit Shelves As Soon As October, Improving Access Nationwide”